Japan Fertilizer & Ammonia Producers Association
History
The Japan Urea & Ammonium Sulphate Industry Association and The Japan Phosphatic & Compound Fertilizers Manufacturers' Association merged to form The Japan Fertilizer & Ammonia Producers Association in July, 2003.Purpose of the association
The Japan Fertilizer & Ammonia Producers Association consists of manufacturers and distributors of fertilizer, fertilizer raw materials, and ammonia and its derived products, who have given consent to the intent of the association. Our main objective is to establish good relationship among the members and to contribute to the expansion of fertilizer and ammonia industries.Activities of Japan Fertilizer & Ammonia Producers Association
- Smooth communication, good relationship, educational opportunities and exchange of information among members.
- Research and studies of fertilizer and ammonia industries.
- Increasing productivity and technological advancement of fertilizer and ammonia industries.
- Research and studies of environment and required countermeasures.
- Research and bulletin on working environment and safety.
- Lobbying activities.
- Public relations.
- Others.
List of members
- Asahi Agria Co., Ltd.
- Asahi Kasei Corp.
- Central Kasei Chemical Co. Ltd.
- Hanwa Co., Ltd.
- JCAM AGRI Co., Ltd.
- Katakura & Co-op Agri Co., Ltd.
- MC Ferticom Co., Ltd.
- Mitsubishi Gas Chemical Company, Inc.
- Mitsui Chemicals, Inc.
- New Chemical Trading Co., Ltd.
- Nihon Hiryo Co., Ltd.
- Nissan Chemical Corporation
- Onoda Chemical Industry Co., Ltd.
- Resonac Corporation
- Sumitomo Chemical Co., Ltd.
- Sun Agro Co., Ltd.
- Taki Chemical Co., Ltd.
- Toagosei Co., Ltd.
- UBE Corporation
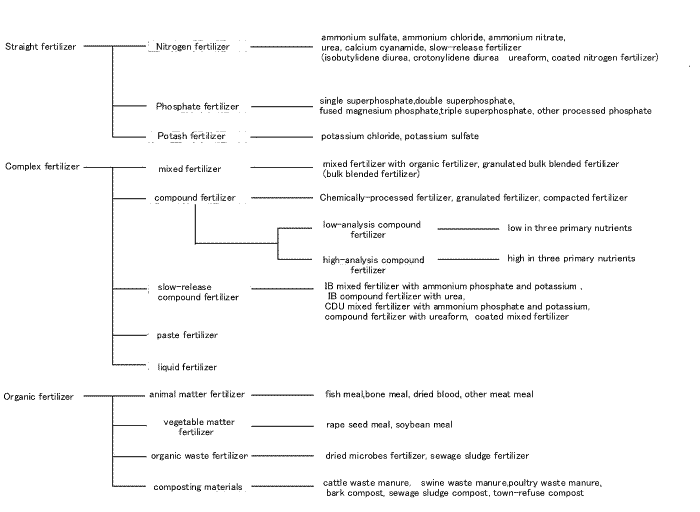
Classification of fertilizers
What is compound fertilizer?
Classification of fertilizers
Fertilizer is classified into organic and inorganic fertilizer ( chemical fertilizer ). Inorganic fertilizer is classified into straight fertilizer ( fertilizer containing single nutrient of nitrogen, phosphate or potassium) and mixed fertilizer ( fertilizer containing more than double nutrients of nitrogen, phosphate or potassium). Compound fertilizer which contains is a form of mixed fertilizer which contain all elements in one granule.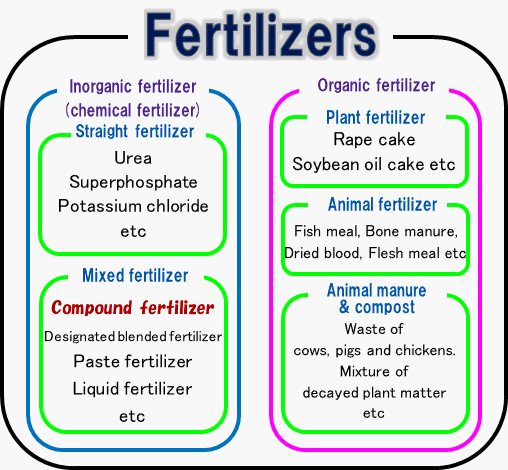
Compound fertilizer
Classification of fertilizers
Compound fertilizer is made from phosphate rock and potash ore which exists in nature. Compound fertilizer contains more than two elements of nitrogen, phosphoric acid or potassium.Compound fertilizer is classified into low-analysis compound fertilizer and high-analysis compound fertilizer according to the total amount of fertilizer elements. The former fertilizer contains 15%~30% of fertilizer elements and the latter fertilizer contains more than 30% of fertilizer elements.
(Example)
| Nitrogen | Phosphate | Potassium | Total amount of elements | Classification |
| 8% | 8% | 8% | 8% | Low-analysis compound fertilizer |
| 14% | 14% | 14% | 42% | High-analysis compound fertilizer |
Meaning of numbers written on a bag of fertilizer.

Each number shows the content ratio of nitrogen, phosphate and potassium from left to right. This example shows that this pack of fertilizer contains nitrogen, phosphate and potassium 14% each.
14-14-14 means that there is 42g of fertilizer elements in 100g content.
From raw materials to the final products of compound fertilizer
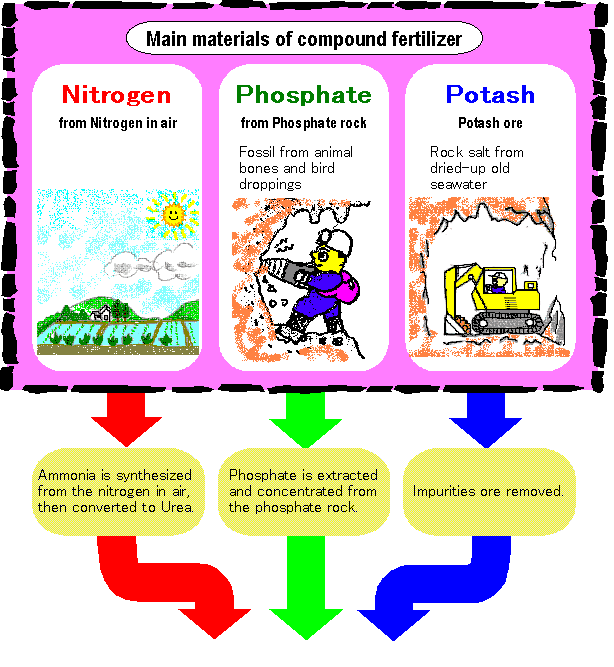
Three element of nitrogen, phosphate
and potassium are granulated through
chemical reaction or steam mixing in
fertilizer plant.
Fertilizer products are sacked in bags
and delivered to the end users.
Compound Fertilizer is well-balanced by nitrogen, phosphate and potassium, easy to handle, reasonable price wise and safe to soil and environment.
- High quality of compound fertilizer is requested the following three points.
- Contains proper effective elements.
- Contain no harmful elements.
- Good physical properties (shape, size, and hardness),which means it is easy to handle.
- Compound fertilizer is not expensive, contains high percentage of effective elements and keeps stable content of nutrients. In addition, the costs of transportation, storing and application are low. Any brand of compound fertilizers is available on demand at any time.
- Besides above good points, compound fertilizer is ready to provide for different variety of crops and soils by changing ratio of nitrogen, phosphate and potassium.
- Compound fertilizers are also suitable for machine application, the trend of market is the increase of so-called functional fertilizers such as controlled release fertilizer (coated fertilizer and flow-in type of fertilizer under the request of labor saving and environment keeping.
Ammonia and nitrogen fertilizer
German scientist Lie big proved plants can grow on inorganic nutrients alone and published his first fertilizer book in 1840. In 1842, Lawes of England began to produce superphosphate and soon succeeded in producing ammoniated superphosphate which was an original form of compound fertilizer.The main nitrogen fertilizer up to early 20th century was sodium nitrate (Chilean saltpeter) discovered in Chile in 1809. However, English scientist Crookes gave his famous speech in 1898 ' As populations grow, the demands for grains would outpace any increase in production as long as we depend on limited resource like such mineral ore. We need to find the way to fix atmospheric nitrogen.' Frank and Caro (German) started to produce calcium cyanamide in Italy in 1906 and then Haber and Bosch succeeded in ammonia synthesis on a commercial basis in 1913, and it was the time modern chemical industry came to blossom.
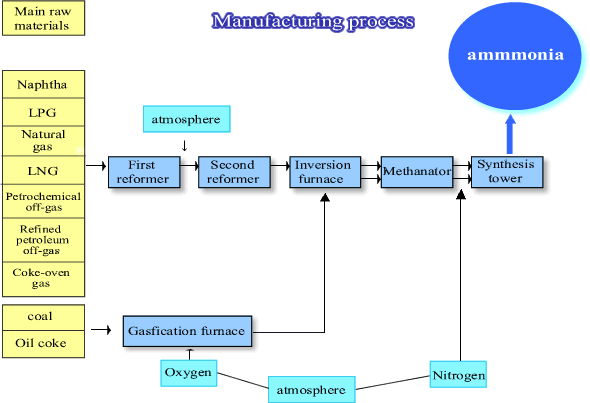
Manufacturing technology of calcium cyanamide was soon adopted in Japan and Shitagau Noguchi and Joichi Fujiyama established The Japan Nitrogen Fertilizer company in 1908 and started to produce it on a commercial basis in 1909. They also introduced ammonia synthesis technology and succeeded in it in Nobeoka plant in 1923. Ammonia synthesis with domestic technology started in 1918 in the Nitrogen Special Research Institute in the Department of Commerce and Agriculture (changed its name to the Tokyo Industrial Laboratory, and then the Chemical Engineering Research Institute), but we had to wait till 1931 to see it successful.
Ammonia synthesis is the technology to combine atmospheric nitrogen with hydrogen to form ammonia. Hydrogen is essential in this process and fossil fuel is essential to produce hydrogen. Synthesized ammonia is used in the production of compound fertilizer and other fertilizers containing nitrogen.
The technology developed by Haber and Bosch was highly evaluated since nitrogen gas is not directly absorbed in crops.
Lime nitrogen is the world's first atmospheric nitrogen fixation of nitrogen-based fertilizer, which does not use ammonia. Lime nitrogen is produced by making calcium carbide with large quantity of electric power and by reaction with nitrogen.
Frank in Germany invented this process in his laboratory in 1989 and its use as fertilizer started as early as in 1901
Ammonia and Derived products flowchart
Ammonium sulphate fertilizer and urea fertilizer can be used as straight fertilizer, though in most cases, with phosphate fertilizer and/or potash fertilizer, they are used as raw materials of complex fertilizer such as compound fertilizer.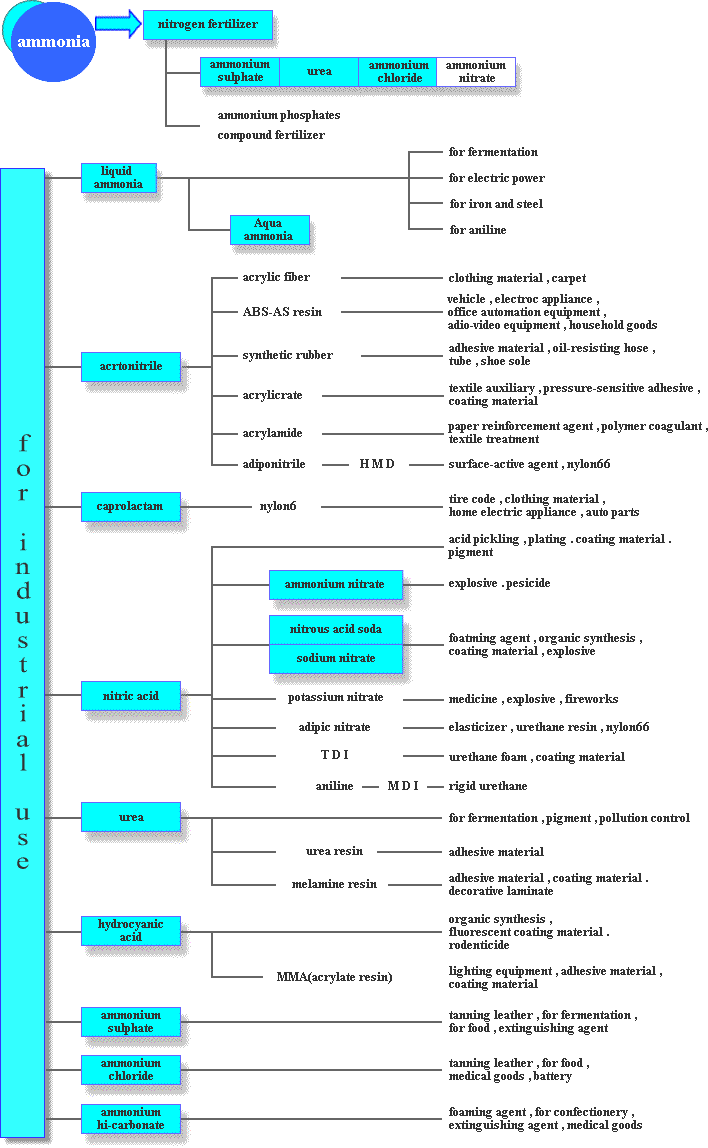
Features of ammonia industry
In most cases, production and consumption of ammonia are within the same plantsite.In foreign countries, most ammonia is used for fertilizer production such as urea and ammonium phosphate. However, under great development of chemical industry in Japan, various kinds of chemical products are efficiently produced within the same plant.
By using various kinds of raw materials, we do our best to maintain stable supply and save resources.
Ammonia industry depends on materials from foreign countries as is the case with the other basic materials industries. However, as ammonia is the basis for many kinds of chemical products in Japan, we diversify materials from foreign countries and make efficient use of by-product off-gas from oil finery and other industries to maintain stable supply.
Ammonia and its derived products
Most of products derived from ammonia are chemical products which have nothing to do with our daily life. However, some of products are controlled by law as they are specified as high-pressure gas, hazardous materials or deleterious substance. The Japan Fertilizer & Ammonia Producers Association has created MSDS written about handling and storing those products and guideline for a warning label on containers to prevent an accident.Ammonium Sulphate
Ordinary ammonium sulphate is crystal or powdery form in white, whereas colorless crystal ammonia sulphate and colored ammonia sulphate are also available. Ammonia sulphate is mainly used for chemical fertilizer such as solo ingredient of nitrogen fertilizer and ingredient of mixed fertilizer. Japan is one of the world's leading ammonia sulphate exporters and in its main market, Southeast Asia, there being lack of sulphur in soil, fertilizer containing sulphur is highly appreciated because it prevents crops from becoming poor quality. In the past, ammonia and sulphuric acid were synthesized directly. Nowadays ammonium sulphate is produced together with caprolactam. Ammonium sulphate is also used for industrial purpose such as cellophane, tanning leather, culture of yeast and food additives.Urea
Ammonia and carbon dioxide(Co2) acid gas are synthesized directly to produce urea. It is prilled in form without any odor or color and slightly hygroscopic. Urea is mainly used for chemical fertilizer such as solo ingredient of nitrogen fertilizer as is ammonium sulphate and ingredient of mixed fertilizer or slow-release nitrogen fertilizer. Dilute urea liquid can be sprayed on leaves as fertilizer and put a little amount into feed for chewers. In addition, it is of wide use of industrial purpose such as plastic, dyeing, medicines and organic synthesis. Urea and melamine can be used either separately or as condensation resin for molding compound, adhesive material, paint, textile-processing and paper converting. By impregnating printing paper with melamine and applying heat and pressure, melamine resin decorated sheet can be produced, which offers great resistance to water and heat and has various uses.Ammonium Nitrate
Ammonium nitrate is crystal in form without any odor or color. Ammonium nitrate solution made by having aqua fortis react to ammonia is concentrated and dried to produce ammonium nitrate. It explodes when mixed with organic matter, heated or shocked. It is used for materials for explosives, fireworks and insecticides as well as materials for mixed fertilizer.Ammonium Bicarbonate
Ammonium bicarbonate is crystal in form without any odor or color, made with ammonia and carbon dioxide. It is used to make baking powder, medicine, extinguishing agent, washing agent, to degrease woolen fabric and to extract rare earthes.Liquid Ammonia Aqua Ammonia
Liquid ammonia is produced when ammonia is put the pressure on and cooled down. The uses of liquid ammonia are ammonia derived products such as nitric acid and urea, materials for caprolactam and acrylonitrile, fermentation for glutamic acid soda, disposal of NOx occurring in the course of thermal power generation and surface treatment for iron (protection against oxidation). Aqua ammonia which is also called ammonium hydroxide is produced by dissolving ammonia in water. The uses of aqua ammonia are medicines, cleaning woolen fabric, cooling medium for refrigerator and solidifying raw rubber.Nitric Acid
Nitric acid is produced by having ammonia react to compressed air or oxygen and then absorbed in water. Nitric acid at this phase is called aqua fortis and when it is concentrated, concentrated nitric. Nitric acid is corrosive liquid which has a characteristic odor and no color or slightly yellowish. It has a variety of uses such as organic synthesis, nitro compound, celluloid, explosive, dyeing, plating, photoengraving and medicines as well as producing intermediate materials (adipic acid, TDI・MDI) for nylon and urethane.Nitrous Acid Soda Sodium Nitrate
Nitrous acid soda and sodium nitrate can be produced by having ammonia oxide or aqua fortis react to caustic soda. Nitrous acid soda is either granular or powder in form with white or slightly yellowish in color. The uses of nitrous acid soda are foaming agent, surface treatment agent for metals, heat treatment agent, dye mordant and organic synthesis. Sodium nitrate is either white powder or clear granular and used for antifoaming agent for glasses, dyeing and explosivesWhat is ammonia?
Ammonia (NH3) is colorless gas at normal temperature and changes into white smoke when released into the atmosphere. Ammonia can be liquefied easily when pressurized or cooled, and liquid form of ammonia is called liquid ammonia. The amount of heat it absorbs from surrounding environment when changing from liquid to gas is the greatest next to water and it dissolves various kind of matters.When ammonia is dissolved in water, it is called aqua ammonia (NH4OH), which is sold in a polyethylene or glass bottle for ordinary users. On the contrary, liquid ammonia is either transported by pipe line or carried by tankship and tanker and sometimes it is put into a cylinder to trade. Because of the explosive property of liquid ammonia when mixed with air or in contact with strong acid, ordinary people rarely see it.
Ammonia is used as a basis to produce various kinds of everyday chemical products. Here are two most popular products of ammonia.
One such kind of product is nylon used for clothing. Nylon is made from caprolactam and ammonia is used as an in-process material. As popular functional plastic, nylon is used for machine parts (a tire cord, a gear and a bearing), auto parts (a bulb for a carburetor, a container for brake fluid), home electric appliances, building materials and medical supplies.
The other kind of product is acrylonitrile made from ammonia and propylene. Acrylonitrile is the main raw material of synthetic rubber, which is called acrylic fiber or NBR. As ABS resin, its properties are hard, strong and easily shiny-colorable, which makes it possible to meet variety of consumers' demands such as home electric appliances, interior and exterior of cars, office automation equipments and housing materials
Ammonia production process

 広報資料
広報資料



